To continue my discussion on water, I need to side-track and discuss starch. Last time I introduced water activity ending up with the comment that:
The reason why the crackers and peanuts get soft after water is absorbed is fascinating to food chemists…
I had already explained that differences in water activity were the reason why peanuts go soft in carrot and peanut salad, and why the same happens with crackers when combined to cheese. It is also true that hard cookies (biscuits for my UK readers) and crackers (cheese biscuits ditto) go soft over time especially in humid places like Delaware. But why, I hear the more intelligent of my readers cry, doesn’t pasta get softer with time. This, my dear readers, is to do with starch.
Starch is two polysaccharides; polymers made up of the monosaccharide, glucose. Glucose, for some unknown* reason, is also known as dextrose by the food industry especially in the US**. The two starch polymers are amylose and amylopectin. Amylose is straight chained and looks like this:

Amylose chains wind into helices:

Amylopectin has branches and looks like this:
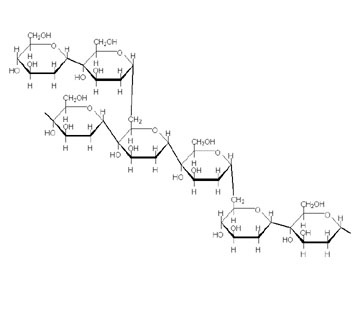
It is thought to look like a tree:

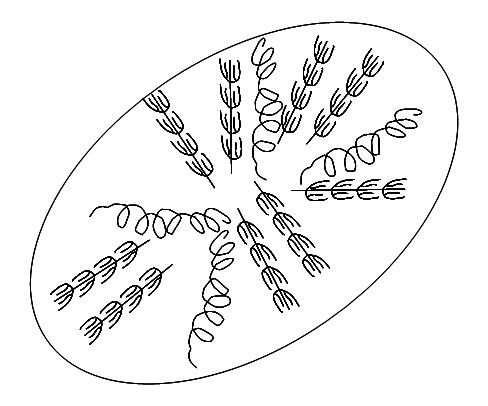
Starch is present in most plants and is used frequently as storage. So seeds and tubers, wheat and potato, have a high percentage of starch. Starch occurs in starch granules. They are many different shapes, depending on the source***:


The ratio of amylose to amylopectin also varies between sources. There is even a source of corn (waxy corn) that contains no amylose. The starch granules are very organized with amylose helices and amylopectin trees:
When starch granules are placed in cold water, nothing really happens. As the water is heated, water is absorbed into the starch granule which, naturally begins to swell. As water absorption and heating continues, the starch granules burst releasing amylose to the outside of the granule and causing the amylopectin to be less ordered. The amylose also loses its helix:

This occurs once the temperature of the starch slurry is above the gelatinization temperature, which is 62 – 70 C for corn starches and 59-67.5 for potato starch.
Once the starch is cooled, if no stirring occurs a gel is formed:

Eventually retrogradation occurs. Anyone who has stored a corn starch custard or gravy in the fridge has seen the effects of retrogradation (and gelation). The starch gel shrinks and a water layer forms on the surface. The release of water during retrogradation is called syneresis. Gelation is caused by the amylose becoming re-ordered and retrogradation is caused by amylopectin reorder.
Gelatinization causes the crystalline structure of the starch granule to be lost, essentially turning the starch molecules into a liquid. Once heat is removed, the starch molecules start to solidify, but they are no longer organized in the same way as they were before cooking. Amylose molecules can therefore sit together, forming tight junctions. If the starch is cooled very rapidly, there will be amorphous domains within in the crystalline superstructure.
As time passes, water absorbed by the amylopectin in the starch granule is released as the molecule also becomes more ordered. This is why syneresis occurs. Eventually the starch molecules will be mostly crystalline.
So what has this to do with why pasta doesn’t absorb water? Unless pasta is heated to above its gelatinization temperature, water is not absorbed by the starch molecules. Dry pasta has been partially heated to remove the water, but the starch granules are typically still unaltered. Thus, water cannot be absorbed until the gelatinization temperature is reached. Biscuits, cookies and crackers, on the other hand, have been thoroughly cooked and the starch granules disrupted. Thus, water can more easily be absorbed. In fact, it is thought that starch, together with the wheat protein – gluten, forms a continuous network in baked products. The staling of bread is the retrogradation of starch, especially amylopectin.
References
Any food chemistry and baking/cereal science textbook
—–
Footnotes:
*Actually the reason it is called dextrose is known – glucose turns polarized light to the right (dex) and fructose to the left. Fructose’s alternative name is laevulose which my dyslexic brain is very grateful that it didn’t catch on. Spelling dyslexia is hard enough. What I don’t understand is why dextrose has stuck. Mind you I’m not sure why the US hasn’t converted to metric either. Probably because the French invented it. But then the US has only recently started hating the French and the Brits have…oh I digress.
**The US/UK language business will be the death of me. Not only are the two countries divided by having the same language (+)– the inside of my brain is too.
(+) I give the credit for this quote to George Bernard Shaw, but it seems that many witty Brits and Americans have said it and get claim credit for it.
***Starch granule pictures from the web:Top is corn starch; bottom is potato starch. All other pictures are my own. I make no apology for them – I never claimed to be an artist. Drawing them was fun, much to my surprise.

Pingback: Tangled Bank Number 64 « The Neurophilosopher’s weblog
Pingback: Physicochemical Properties of Starch « Lab Cat
Have you tried to leave pasta on cold water for a few hours? It does absorb water. The amylose does not get dissolved in water, but the pasta has definitely absorbed water. What would bde the explanation for this?
Pingback: CPI (Curioso pero inútil) » Blog Archive » Consultorio CPI: Cocinando en altura
John
Pasta can absorb water as it is partially cooked and some of the starch granules have been disrupted during the making of pasta.
It would be the same process as for cooked pasta but not all the amylose and amylopectin will gelatinize as the temperature is not high enough.
Now I have to try it and see how soft it gets.
At last someone was able to explain to me the behavior of starch in simple terms! Thank you for that! and your drawings are sublime too!
Now, how can I use this knowledge to make better colombian arepas*? They have to be crusty, slightly golden tone and with grill marks on the outside and creamy and tender inside. Mine tend to be crusty throughout.
*1- corn is partially degermed and partially removed cuticle by mechanical means (not nixtamalization). 2- Cooked in boiling water for 3 hours 4- milled in a meat mill 5- formed into round 15 cm diameter and 1 cm thick arepas weighing 123 g. 6- Grilled over hot plates until it reaches 80°C inside and the weigh is reduced to 100 g. 7- Stored under refrigeration. Heated again over the stove before eating. Aw at 23.1°C is 0.99 (molds are a headache). Moisture content is 64%. pH 5.3.
It is obvious that I am overcooking the starch and killing its water binding ability, raising Aw. Someone told me that the meat mill with 2 mm hole disk is producing a paste with large particles that allow the water to escape from the dough inside leaving the arepa crusty throuhgout.
How to cook the corn properly? perhaps grinding the kernels to a certain mesh, add hot water to reach 70°C, obtain a play-dough type texture to mold the arepas.
As odd as it sounds, here in Colombia we eat arepas every day of our lives and nobody knows how to make a perfect arepa! Any hints? can you shed some light into this problem?
Wow, I’m glad I found this. Your explanation of starch retrogradation and gelation is going to help me on my food chem test. Thanks, Dr. LabCat!
are carrots possible to manufacture starch?
Carrots contain starch.
Your write up are good but pls help us to go more deeper in the area of starch especially carbohydrates
Pingback: Nutrimenti » Blog Archive » Amido resistente in cucina