Perviously I have discussed water’s nutritional properties and how much we should be drinking. This post is a beginning of a series on water chemistry. I’ve been putting off this post because water chemistry is so complicated. Fascinating but complicated.
The easy bit. Hopefully most people realise that water is made up of two chemicals, hydrogen (H) and oxygen (O). There are two hydrogen molecules for each oxygen so water can be represented as H2O.
A single water molecule is typically drawn looking a bit like Mikey Mouse with grey hydrogen ears and blue face:

Even after being bonded to hydrogen, the oxygen has two unpaired electrons. This means that oxygen is electronegative and so the oxygen atom of water has a partial negative charge. Since this isn’t a full negative charge it is commonly represented by delta -. Hydrogen has a partial positive charge. This gives water a dipolar nature. 
This dipolar nature causes a hydrogen from one molecule of water to be attracted to an oxygen of another ending up with some kind of network linking the water molecules together. The bonds between oxygen and hydrogen are called hydrogen bonds and there can be up to four hydrogen bonds per water molecule. My version of what a water network might look like on a molecular scale is below, but no one really knows.
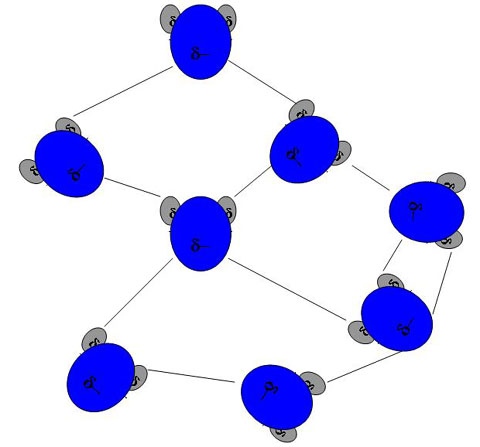
You have to imagine also that this is a 3D network with hydrogen bonds coming both into the screen and out towards you. After all water has volume and is more than one layer of molecules thick.
Why are hydrogen bonds so important? Hydrogen bonding in water is one of the reason why water has its unique properties. For a molecule of its size – it has a molecular weight of 18; it has very high melting and boiling points. A molecule of a similar size, Methane, with a molecular weight of 16 has a melting point of −182.5°C and a boiling point of −161.6°C. Water melts at 0°C and boils at 100°C. The reason these transition temperatures are so much higher in water is that more energy is required to convert ice into water and water into steam than required to convert methane from a solid to a liquid and then to a gas. Even though the hydrogen bonds themselves are relatively weak, breaking them requires energy. This conveniently means that water is a liquid at the temperatures most of us live. Without this property of water perhaps life would not have occurred. It certainly wouldn’t be in the form it is on Earth today.
Hydrogen bonding causes other unique properties of water, which are also essential for life, but you’ll just have to wait for another time.

Pingback: See You at Enceladus » Blog Archive » Blog Carnivals Round-Ups #1
Pingback: Lab Cat » Blog Archive » Water is Unique
what effect does hydrogen bonding have on the properties of water?
Bill
You need to visit the next article in the water series. The link is above – it is called “Water is Unique”.
Cat
H bonding is polar ( H has partial + charge, O has – charge) this create strong interative forces while methane ch4 does not have/ or is not regarded has having such forces. this is because methan is symetrical while water is not.
you should also look at your periodic table where is the carbon of (methane) in comparison to oxygen of water in electronegativeity series,
Andy
Sigh. Did you even read the post?
What causes Hydrogen bonding? Can we do it here on earth? Can we physically make water by initiating a H2O bond to make water molecules? If Hydrogen atoms and Oxygen atoms came from exploding stars, then what action occured to make them attract or Hydrogen bond? I can’t find an answer anywhere. Can anyone help?
Steve
I’m not sure what you are after. Hydrogen bonding happens due to the fact that oxygen has unpaired electrons and is therefore electronegative. This is caused by the way the electrons are arranged around the oxygen atom and is part of their fundamental nature.
My work suggests that the back of the oxygen contains a pit into which the hydrogen of another water can snuggel up. The density of electric charge increases with the release of energy. This brief hint has the oxygen with one back pit for one hydrogen so a water can participate in at most three H bonds, but an average of 2 and when hot, less than that.
Aah cute, oxygen atoms have dimples that hydrogen atoms like to cuddle up with.
Really?
Can we make drinking water by chemical reaction of hydrogen and oxygen?
Could I have the name of the author of this article? I am doing background research for a science fair project and would greatly appreciate it.
Thanks!
What are the chemical bonding possibilities of hydrogen!!
Please answer this!
Also what other molecules are their of hydrogen other than H2O!
MOE 😀
My names dillon I Know this question isnt part of hydrogen nor oxygen but could you please help me.
( This is about carbon)
How Does Carbon React With Other substances??????????????????????
Thanks if you help me 😀 😛 :O
UR all frkn nerds HAHAHA LOL
Can hydrogen and oxygen be mixed to produce water at a specified temperature and pressure?